|
|
| (29 intermediate revisions by 4 users not shown) |
| Line 1: |
Line 1: |
| − | ==<font color=red> Public Comment on QIBA Profiles and Protocols</font>==
| + | [[Image:QIBA_WIKI_BLURB.jpg|600px]] |
| | | | |
| | + | <font color="red">NOTICE: These instructions are accessible on the QIBA Wiki as a historical reference only! Independent Profile Authoring Groups are required to oversee the various developmental stages from Draft to Public Comment to Consensus based on the current QUIC endorsement criteria, found HERE:</font> https://qibawiki.rsna.org/images/9/95/QUIC-Review-Criteria-for-New-Profiles-FINAL-v23SEPT2023.pdf. |
| | | | |
| − | All QIBA technical documents (Profiles, Protocols) are posted for Public Comment to ensure stakeholder representation, transparency and quality review. All documents are posted via the QIBA WIKI and announced to a broad e-mail distribution list. QIBA documents are posted for public review for a minimum of 30 days before being considered finalized for trial implementation.
| |
| | | | |
| | + | QIBA '''[[Profiles|Profile documents]]''' are posted for '''[[Public Comment Process|Public Comment]]''' to ensure stakeholder representation, transparency and quality review. Interested people are encouraged to provide input on the document, the scenarios and use cases they address, the technologies proposed and other details. |
| | | | |
| − | The Public Comment Phase of the development of each QIBA Profile/Protocol allows interested people to provide input on the document, the scenarios and use cases they address, the technologies proposed and other details. In particular, vendors who will implement the Profiles/Protocols and members of the healthcare community who will use the Profiles/Protocols are encouraged to review and comment.
| + | ==Comment Submission== |
| | | | |
| − | *Review the documents listed in the Public Comment WIKI page | + | * ** provide a link to a public comment form (Google forms are recommended for ease of data capture) - ''see example'': [[Image:public comment form.jpg |1200px]] |
| − | *Note the deadline listed next to each document | + | :* Select the document you are commenting on from the appropriate form |
| − | *Submit your comments via the comment form or direct e-mail to ([mailto:sweinmann@rsna.org Susan Weinmann]) | + | :* Provide your name and email address |
| | + | :* Add the Profile section and line number (if applicable) to which your comment refers |
| | + | :* Describe the issue and your propose a resolution |
| | + | :* When you have entered each of your comments, you may submit the form |
| | + | * e-mail Profile Authoring Group with ''general comments'' about the Profile/Protocol concept, structure, process, etc. |
| | | | |
| | + | The [[Committees|QIBA Biomarker Committees]] consider all comments submitted, and where appropriate, post responses. |
| | | | |
| − | The QIBA Biomarker Committees who develop QIBA Profiles and Protocols will consider all comments submitted in the specified public comment period, and where appropriate, respond to comments. Please see below for more detailed instructions for submitting comments.
| + | ==Public Comment Documents== |
| | | | |
| − | ==Directions for Comment Submission==
| + | see http://qibawiki.rsna.org/index.php/Profiles |
| − | | |
| − | Use the '''[https://fs22.formsite.com/QIBA/QIBA_SPECT_BC_Profile/index.html]''']] to submit comments concerning QIBA Profiles/Protocols to the QIBA Biomarker Committees. Documents currently in the Public Comment phase are listed below. Submitted comments will be considered for the revised versions of these documents.
| |
| − | | |
| − | * Please insert your comments on specific Profile/Protocol content into the downloadable [[Media:QIBA Public Comment Form3.doc|'''Public Comment Form''']] and send by e-mail attachment to ([mailto:sweinmann@rsna.org Susan Weinmann])
| |
| − | | |
| − | * If you have ''general comments'' about the Profile/Protocol concept, structure, process, etc., please e-mail those directly to ([mailto:sweinmann@rsna.org Susan Weinmann])
| |
| − | | |
| − | | |
| − | '''<big><font color=red> Instructions: </font></big>'''
| |
| − | | |
| − | 1. Select and download Profiles or Protocols for review (see link below).
| |
| − | | |
| − | *Profile:
| |
| − | *Protocol:
| |
| − | | |
| − | 2. Designate '''Section''' or '''Line Number''' for each comment (or designate as "general").
| |
| − | | |
| − | 3. Specify the '''Issue''' concisely and provide any new or replacement text in the '''Proposal''' section.
| |
| − | | |
| − | 4. Designate the '''Priority''' of each comment as High ('''H'''), Medium ('''M'''), or Low ('''L''')
| |
| − | * H: Important issue where there is a major issue to be resolved; requires discussion and debate.
| |
| − | * M: Medium issue or clarification. Requires discussion, but should not lead to long debate.
| |
| − | * L: Typo or other minor classification that an editor can manage; requires no group discussion.
| |
| − | | |
| − | 5. After completing form, save it and send as an e-mail attachment to ([mailto:sweinmann@rsna.org Susan Weinmann])
| |
| − | | |
| − | 6. Comments are due by date listed next to each document.
| |
| − | | |
| − | ==<font color=red> QIBA Profiles and Protocols for Reference </font>==
| |
| − | | |
| − | *[[Media:001.QIBA SPECT Profile.v0.1 2016.10.28.docx | QIBA Profile: <big><font color=red> QIBA Profile: Quantifying Dopamine Transporters with 123 - Iodine Labeled Ioflupane in Neurodegenerative Disease Profile (v1.0), October 28, 2016</font></big>]] ''The Public Comment period ends February 1, 2017.''
| |
| − | | |
| − | *[[Media:002.QIBA SPECT Profile Checklist Extract.2016 10 31.docx|QIBA SPECT Profile Conformance Checklist]]
| |
| − | | |
| − | | |
| − | *[[Media:QIBA_CT_Vol_LungNoduleAssessmentInCTScreening_v5-2-16b.docx|QIBA Profile: <big><font color=red> QIBA Profile: Lung Nodule Volume Assessment and Monitoring in Low Dose CT Screening, June 1, 2016</font></big>]] ''The Public Comment period has closed''
| |
| − | | |
| − | *[[Media:QIBA Small Nodule Profile References - Accuracy.xlsx|QIBA Spreadsheet: <big><font color=red> Small Nodule Profile References_Accuracy. Version for Public Comment. June 1, 2016 </font></big>]] ''The Public Comment period has closed''
| |
| − | | |
| − | *[[Media:QIBA Small Nodule Profile References - Precision.xlsx|QIBA Spreadsheet: <big><font color=red> Small Nodule Profile References_Precision. Version for Public Comment. June 1, 2016 </font></big>]] ''The Public Comment period has closed''
| |
| | | | |
| | | | |
| | *[[Document Archive]] | | *[[Document Archive]] |

NOTICE: These instructions are accessible on the QIBA Wiki as a historical reference only! Independent Profile Authoring Groups are required to oversee the various developmental stages from Draft to Public Comment to Consensus based on the current QUIC endorsement criteria, found HERE: https://qibawiki.rsna.org/images/9/95/QUIC-Review-Criteria-for-New-Profiles-FINAL-v23SEPT2023.pdf.
QIBA Profile documents are posted for Public Comment to ensure stakeholder representation, transparency and quality review. Interested people are encouraged to provide input on the document, the scenarios and use cases they address, the technologies proposed and other details.
- ** provide a link to a public comment form (Google forms are recommended for ease of data capture) - see example:
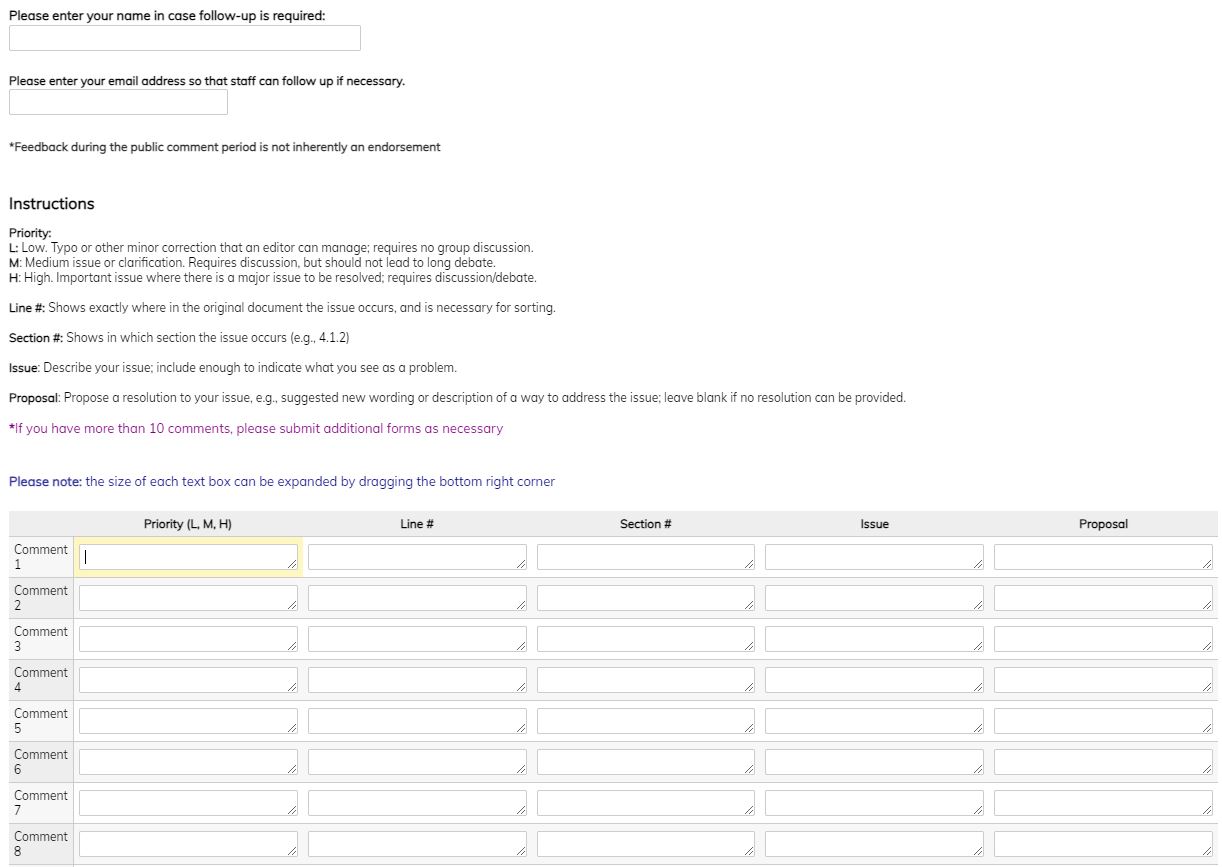
- Select the document you are commenting on from the appropriate form
- Provide your name and email address
- Add the Profile section and line number (if applicable) to which your comment refers
- Describe the issue and your propose a resolution
- When you have entered each of your comments, you may submit the form
- e-mail Profile Authoring Group with general comments about the Profile/Protocol concept, structure, process, etc.
The QIBA Biomarker Committees consider all comments submitted, and where appropriate, post responses.
see http://qibawiki.rsna.org/index.php/Profiles

