PET Tau Biomarker Committee
PET Tau Biomarker Committee
- Co-chairs: Tammie Benzinger, MD, PhD; Dawn Matthews, MS, MBA
- RSNA Staff Support: Julie Lisiecki
Project Snapshot
- Develop a quantitative imaging Profile for PET Tau.
- This effort uses the QIBA Process for Profile development.
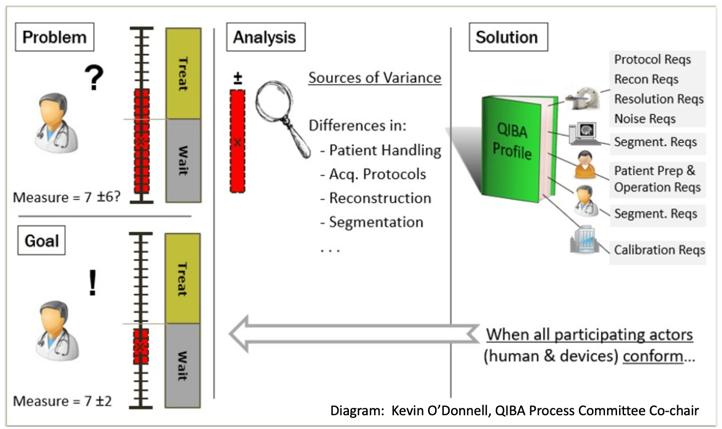
Overview The Quantitative Imaging Biomarkers Alliance (QIBA) was formed with the mission to improve the value and practicality of quantitative imaging biomarkers by reducing variability across devices, sites, patients, and time. This arose from the realization that as quantitation was increasingly used for radiologic measurement in the clinic, wide variability detracted from the meaning and value of numeric results. Outputs are Profile documents that specify parameters for site/equipment qualification, patient preparation, image acquisition, processing, and analysis that enable achievement of a Claim. A Claim is a statement regarding the technical performance that can be achieved through Profile compliance, for example: a change in value can be considered real if it exceeds a stated threshold where starting values are between a certain range; or the coefficient of variability for a given PET tracer in longitudinal measurement is less than a stated value, which enables calculation of required number of subjects for statistical powering. An Amyloid PET Profile was recently released and work toward a Tau PET Profile is initiating based upon interest from academic and industry stakeholders. The committee would like to coordinate and harmonize with other initiatives to which this effort can be complementary. QIBA work follows a defined process: (1) Identify sources of error and variation in image quantitation; (2) Specify potential strategies for error mitigation, and document these in the form of Profiles; (3) Test the feasibility and efficacy of the QIBA Profiles; (4) Disseminate the information. A Profile goes through five stages during which a draft is created and provided for public comment, input is incorporated, several sites perform the Profile steps to confirm Technical feasibility, and larger studies confirm the clinical validity of the claims.
QIBA Profile Fit with Other Initiatives QIBA profiles are intended to be complementary to, rather than redundant or in conflict with, other initiatives and studies designed to harmonize measurements across PET tracers and to optimize measurement. Using published literature and additional confirmatory studies, a QIBA profile identifies that when controlling tracer administration, equipment calibration, head motion management, and other parameters, the expected variability (an input to true change or required numbers of study subjects) is “x”. For example, the Amyloid PET Profile does not dictate which regions of interest or reference region must be used; however, it indicates the CoV achievable for longitudinal measurement using typical cortical regions and if reference region variability (stability) is within that of referenced published approaches (e.g., white matter, Chen et al, 2015). QIBA Tau PET Profile Status The Tau PET Profile committee has been formed and continues to expand with additional interested participants. Elements shared with the Amyloid PET profile (e.g., management of subject head motion, equipment calibration, image alignment and application of regions of interest to minimize technical error) have been migrated in an updated, more streamlined Profile format as groundwork. Initial decisions have been made to include [18F]flortaucipir, [18F]MK-6240, [18F]PI-2620, [18F]RO-948, and [18F]GTP1, focus on the Alzheimer’s disease indication for this version, and emphasize SUVR as the primary measure but with inclusion of DVR discussion and potentially a DVR-focused claim if performance differs. Test-retest literature has been identified and studies that have measured longitudinal variability in cognitively unimpaired amyloid negative subjects are being explored to further support non-binding related variability assessment as was done with amyloid. Profile approaches will consider that tau PET tracers vary considerably with regard to off-target binding and specificity, and that tau accumulates in variable spatial patterns, with different clinical and accumulation rate implications depending upon age, stage, and distribution. Next steps involve evaluation of approaches to take for claim definition and support, methods, and interactions with other efforts
Meetings
PET Tau Call Summaries Archive