Difference between revisions of "VolCT - Group 3A"
| (48 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
==<font color=blue>Challenges that Characterize Variability due to Algorithm Use</font>== | ==<font color=blue>Challenges that Characterize Variability due to Algorithm Use</font>== | ||
| − | ==Invitation to participate in the QIBA 3A CT Volumetry Study of CLINICAL data== | + | ==QIBA 3A CT Volumetry Study of Synthetic Lesions data== |
| + | Sent: Thursday, August 11, 2016 | ||
| + | To: QIBA | ||
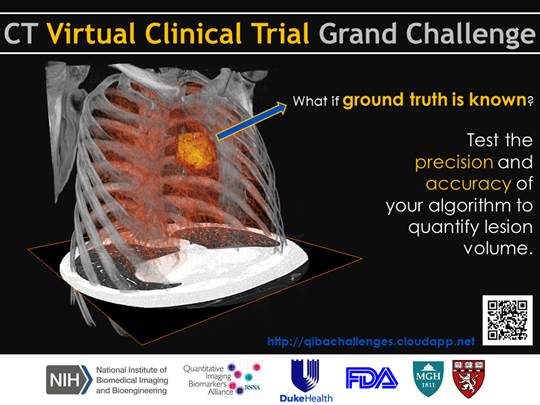
| + | Subject: '''Invitation to Participate in the CT Virtual Clinical Trial Grand Challenge''' | ||
| + | |||
| + | Dear QIBA members: | ||
| + | |||
| + | You are invited to participate in the CT Virtual Clinical Trial Grand Challenge hosted by QIBA in conjunction with ''Duke Health'', the ''US Food and Drug Administration'', and ''Massachusetts General Hospital''. | ||
| + | The overall objective of this challenge is to quantitatively benchmark the volume estimation performance of segmentation tools for lung lesions using phantom and clinical cases. | ||
| + | The aim is to provide a quantitative understanding of the differences between segmentation approaches. | ||
| + | |||
| + | This will involve performing image-based segmentation on datasets generated using: | ||
| + | |||
| + | (1) an anthropomorphic phantom with synthetic and virtually inserted nodules and | ||
| + | (2) clinical images containing real lung lesions and virtually inserted lesion models. | ||
| + | Nodules were virtually inserted using three insertion methods: Techniques A, B, and C, where Technique A is a projection-domain insertion method, and Techniques B and C are image-domain insertion methods. | ||
| + | |||
| + | *Each participant is to use their segmentation algorithm to perform volume estimation on nodules with locations that are a priori provided. | ||
| + | *Participants are encouraged to use their preferred segmentation work flow, and if possible, provide a NIfTI formatted segmentation map used for their morphological characterizations. | ||
| + | *All data will be de-identified, and analyzed in terms of accuracy and precision of estimated volumes. | ||
| + | *Individual outcomes from each algorithm and anonymized aggregate data from all algorithms will be provided to the individual participants for developmental use. | ||
| + | *The aggregate data will be used as a gauge of quantitative variability across segmentation methods. | ||
| + | *<font color=red>The challenge will be active July 26 – November 1, 2016.</font> *<font color=red>Extended to February 28, 2017</font> | ||
| + | |||
| + | This challenge is currently '''LIVE''' and can be accessed at: http://qibachallenges.cloudapp.net/ | ||
| + | ''Sent on behalf of the QIBA CT Volumetry, Group 3A Challenge Organizers'' | ||
| + | |||
| + | *[[Image: 3A Challenge ad 1.jpg]] | ||
| + | |||
| + | |||
| + | ==QIBA 3A CT Volumetry Study of CLINICAL data== | ||
*[[Media:QIBA Volumetric Study 3A-RSNA-2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Overview]] ''posted 04.16.2013'' | *[[Media:QIBA Volumetric Study 3A-RSNA-2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Overview]] ''posted 04.16.2013'' | ||
*[[Media:Invitation Message-updated 6.03.2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Invitation]] ''re-posted 06.04.2013'' | *[[Media:Invitation Message-updated 6.03.2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Invitation]] ''re-posted 06.04.2013'' | ||
*[[Media:Application to participate in the QIBA 3A CT Volumetry Clinical Study-06.04.2013.doc|Application to participate in the QIBA 3A CT Volumetry Clinical Study 2013]] ''re-posted 06.04.2013 (Word doc)'' | *[[Media:Application to participate in the QIBA 3A CT Volumetry Clinical Study-06.04.2013.doc|Application to participate in the QIBA 3A CT Volumetry Clinical Study 2013]] ''re-posted 06.04.2013 (Word doc)'' | ||
*[[Media:InfoFilesandFormats.pdf|Info Files and Formats (Dr. Danagoulian)]] ''posted 04.16.2013 (PDF)'' | *[[Media:InfoFilesandFormats.pdf|Info Files and Formats (Dr. Danagoulian)]] ''posted 04.16.2013 (PDF)'' | ||
| − | *[[Media:UploadingInstructions.pdf|Instructions for Uploading Data to QI-Bench (Dr. Danagoulian)]] | + | *[[Media:UploadingInstructions.pdf|Instructions for Uploading Data to QI-Bench (Dr. Danagoulian)]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Study Design Documents== | ==Study Design Documents== | ||
| Line 19: | Line 43: | ||
3A study design - for proposed clinical challenge | 3A study design - for proposed clinical challenge | ||
*[[Media:3A study design, second challenge, 0.3.PDF|QIBA Volumetric CT 3A Study Design, second challenge, 0.3]] | *[[Media:3A study design, second challenge, 0.3.PDF|QIBA Volumetric CT 3A Study Design, second challenge, 0.3]] | ||
| + | |||
3A study design 1.0.doc | 3A study design 1.0.doc | ||
| − | |||
*[[Media:3A study design 1.0.doc |QIBA Volumetric CT 3A Study Design, 1.0]] '' posted 01.26.2012 for review '' | *[[Media:3A study design 1.0.doc |QIBA Volumetric CT 3A Study Design, 1.0]] '' posted 01.26.2012 for review '' | ||
*[[Media:3A study design 0.9.doc |QIBA Volumetric CT 3A Study Design, 0.9]] '' posted 01.12.2012 for review '' | *[[Media:3A study design 0.9.doc |QIBA Volumetric CT 3A Study Design, 0.9]] '' posted 01.12.2012 for review '' | ||
*[[Media:3A study design 0.7.pdf |QIBA Volumetric CT 3A Study Design, 0.7 (PDF)]] '' posted 9.22.2011 for review '' | *[[Media:3A study design 0.7.pdf |QIBA Volumetric CT 3A Study Design, 0.7 (PDF)]] '' posted 9.22.2011 for review '' | ||
| + | [[Archived Versions of QIBA Vol CT - Group 3A draft documents]] | ||
| − | |||
==Reference Materials== | ==Reference Materials== | ||
| + | *[[Media:QIBA 3A Group FEATURE WORK DEcember 2014 MA.pdf|QIBA Group 3A Feature Work Overview]] ''posted 12.16.2014'' | ||
*[[Media:QIBA Volumetric Study 3A-RSNA-2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Overview]] ''posted 04.02.2013'' | *[[Media:QIBA Volumetric Study 3A-RSNA-2013.pdf|QIBA Volumetric CT 3A Clinical Challenge Overview]] ''posted 04.02.2013'' | ||
*[[Media:QIBA Volumetric CT 3A Group meeting on 03.03.2011 PPT.pdf|QIBA Volumetric CT 3A PowerPoint slides from meeting on 03.03.2011]] '' posted 3.14.2011 '' | *[[Media:QIBA Volumetric CT 3A Group meeting on 03.03.2011 PPT.pdf|QIBA Volumetric CT 3A PowerPoint slides from meeting on 03.03.2011]] '' posted 3.14.2011 '' | ||
*[[Media:QIBA Volumetric CT 3A Group first meeting-20110113.pdf|QIBA Volumetric CT 3A Group First Meeting]] | *[[Media:QIBA Volumetric CT 3A Group first meeting-20110113.pdf|QIBA Volumetric CT 3A Group First Meeting]] | ||
*[[Media:Gavrielides_OE_posted.pdf|Gavrielides et al. Phantom data set acquisition procedure paper]] '' posted 2.5.2013'' | *[[Media:Gavrielides_OE_posted.pdf|Gavrielides et al. Phantom data set acquisition procedure paper]] '' posted 2.5.2013'' | ||
| + | *[[Media:QIBA Volumetric Study 3A Pivotal-Pilot1.pdf | QIBA Volumetric Study 3A Final Pilot and Pivotal Studies {Part I}]] | ||
| + | *[[Media:QIBA Volumetric Study 3A Pivotal-Pilot2.pdf | QIBA Volumetric Study 3A Final Pilot and Pivotal Studies {Part II}]] | ||
| − | |||
| − | + | ==Study Summaries== | |
| − | + | *[[Media:QIBA Volumetric Study 3A Pilot-Summary.pdf | QIBA Volumetric Study 3A Final Pilot Summary]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | *[[Media: | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ==Call Summaries== | |
| − | + | '''''[[Group 3A Call Summaries Archive]]''''' | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==WIKI help protocols== | ==WIKI help protocols== | ||
*[[Media:Microsoft Word-Wiki Protocol-v2.pdf|Using the WIKI]] | *[[Media:Microsoft Word-Wiki Protocol-v2.pdf|Using the WIKI]] | ||
*[[Media:Wiki Help-History Overview2.pdf|Using the WIKI to check history and compare documents]] | *[[Media:Wiki Help-History Overview2.pdf|Using the WIKI to check history and compare documents]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 21:40, 12 December 2017
Challenges that Characterize Variability due to Algorithm Use
QIBA 3A CT Volumetry Study of Synthetic Lesions data
Sent: Thursday, August 11, 2016 To: QIBA Subject: Invitation to Participate in the CT Virtual Clinical Trial Grand Challenge
Dear QIBA members:
You are invited to participate in the CT Virtual Clinical Trial Grand Challenge hosted by QIBA in conjunction with Duke Health, the US Food and Drug Administration, and Massachusetts General Hospital. The overall objective of this challenge is to quantitatively benchmark the volume estimation performance of segmentation tools for lung lesions using phantom and clinical cases. The aim is to provide a quantitative understanding of the differences between segmentation approaches.
This will involve performing image-based segmentation on datasets generated using:
(1) an anthropomorphic phantom with synthetic and virtually inserted nodules and (2) clinical images containing real lung lesions and virtually inserted lesion models. Nodules were virtually inserted using three insertion methods: Techniques A, B, and C, where Technique A is a projection-domain insertion method, and Techniques B and C are image-domain insertion methods.
- Each participant is to use their segmentation algorithm to perform volume estimation on nodules with locations that are a priori provided.
- Participants are encouraged to use their preferred segmentation work flow, and if possible, provide a NIfTI formatted segmentation map used for their morphological characterizations.
- All data will be de-identified, and analyzed in terms of accuracy and precision of estimated volumes.
- Individual outcomes from each algorithm and anonymized aggregate data from all algorithms will be provided to the individual participants for developmental use.
- The aggregate data will be used as a gauge of quantitative variability across segmentation methods.
- The challenge will be active July 26 – November 1, 2016. *Extended to February 28, 2017
This challenge is currently LIVE and can be accessed at: http://qibachallenges.cloudapp.net/ Sent on behalf of the QIBA CT Volumetry, Group 3A Challenge Organizers
QIBA 3A CT Volumetry Study of CLINICAL data
- QIBA Volumetric CT 3A Clinical Challenge Overview posted 04.16.2013
- QIBA Volumetric CT 3A Clinical Challenge Invitation re-posted 06.04.2013
- Application to participate in the QIBA 3A CT Volumetry Clinical Study 2013 re-posted 06.04.2013 (Word doc)
- Info Files and Formats (Dr. Danagoulian) posted 04.16.2013 (PDF)
- Instructions for Uploading Data to QI-Bench (Dr. Danagoulian)
Study Design Documents
3A study design - for proposed clinical challenge
3A study design 1.0.doc
- QIBA Volumetric CT 3A Study Design, 1.0 posted 01.26.2012 for review
- QIBA Volumetric CT 3A Study Design, 0.9 posted 01.12.2012 for review
- QIBA Volumetric CT 3A Study Design, 0.7 (PDF) posted 9.22.2011 for review
Archived Versions of QIBA Vol CT - Group 3A draft documents
Reference Materials
- QIBA Group 3A Feature Work Overview posted 12.16.2014
- QIBA Volumetric CT 3A Clinical Challenge Overview posted 04.02.2013
- QIBA Volumetric CT 3A PowerPoint slides from meeting on 03.03.2011 posted 3.14.2011
- QIBA Volumetric CT 3A Group First Meeting
- Gavrielides et al. Phantom data set acquisition procedure paper posted 2.5.2013
- QIBA Volumetric Study 3A Final Pilot and Pivotal Studies {Part I}
- QIBA Volumetric Study 3A Final Pilot and Pivotal Studies {Part II}
Study Summaries
Call Summaries
Group 3A Call Summaries Archive