Difference between revisions of "QIBA Profile Conformance"
Jump to navigation
Jump to search
| Line 12: | Line 12: | ||
== <font color=blue>Before starting conformance testing, please </font> [mailto:qiba@rsna.org Email QIBA] == | == <font color=blue>Before starting conformance testing, please </font> [mailto:qiba@rsna.org Email QIBA] == | ||
| − | == <font color=purple>''' | + | == <font color=purple>'''Certification Service for Conformance'''</font> == |
===CT Small Lung Nodule Volumetry for Screening=== | ===CT Small Lung Nodule Volumetry for Screening=== | ||
| Line 23: | Line 23: | ||
The following QIBA Profiles have reached the Consensus Stage or the Technically Confirmed Stage, and are thus appropriate for self-attestation conformance testing. | The following QIBA Profiles have reached the Consensus Stage or the Technically Confirmed Stage, and are thus appropriate for self-attestation conformance testing. | ||
| + | |||
| + | See '''[[Profile Conformance]]''' | ||
===CT: Lung Tumor Volume (Advanced Disease)=== | ===CT: Lung Tumor Volume (Advanced Disease)=== | ||
Revision as of 00:26, 10 April 2020
Self-attestation (Registered) or QIBA Tested (Certified) Opportunities
{This content will eventually be available on the RSNA.org website.}
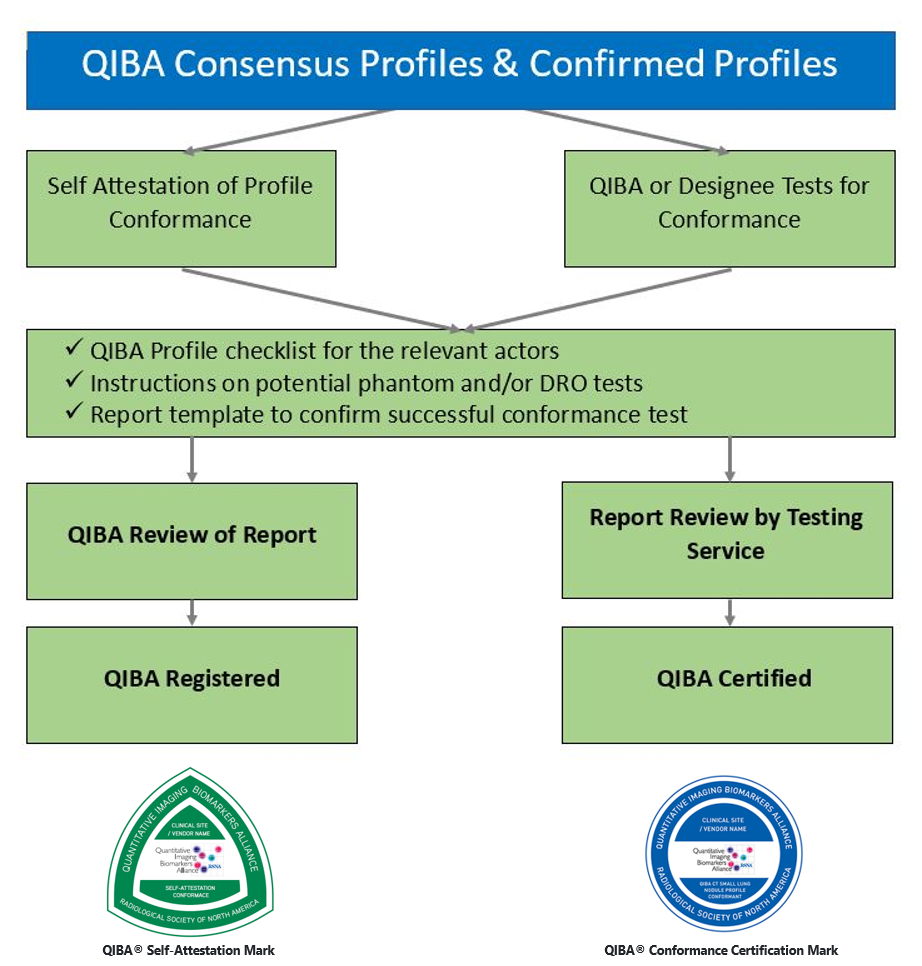
QIBA Profiles that are at least at the Consensus Stage are available to interested parties (clinical sites, CROs, equipment and/or software vendors) to demonstrate that all relevant actors of an institution or product conform to the respective QIBA Profile.
As shown in the following diagram, QIBA Profile conformance may be demonstrated via Self Attestation, or (when available) via a Certification service:
Before starting conformance testing, please Email QIBA
Certification Service for Conformance
CT Small Lung Nodule Volumetry for Screening
- CT Small Lung Nodule Volume Assessment and Monitoring in Low Dose Screening 2018-11-18
- https://www.rsna.org/en/research/quantitative-imaging-biomarkers-alliance/qiba-conformance-certification-services
Self Attestation of Conformance
The following QIBA Profiles have reached the Consensus Stage or the Technically Confirmed Stage, and are thus appropriate for self-attestation conformance testing.
CT: Lung Tumor Volume (Advanced Disease)
Profile
Checklist
- CT Conformance Checklist (can also refer to Appendix C in the Profile document - pg 42)
- Assessment Procedures (refer to Section 4 in the Profile document - pg 30)
Report Template
FDG-PET: Solid Tumor SUV
Profile
Checklist
Report Template
DWI MR: ADC of Lesions in Four Organs
Profile
Checklist
Report Template