Pilot Conformance Projects Under Development
Jump to navigation
Jump to search
The printable version is no longer supported and may have rendering errors. Please update your browser bookmarks and please use the default browser print function instead.
QIBA Profile Conformance
{This content will eventually be available on the RSNA.org website.}
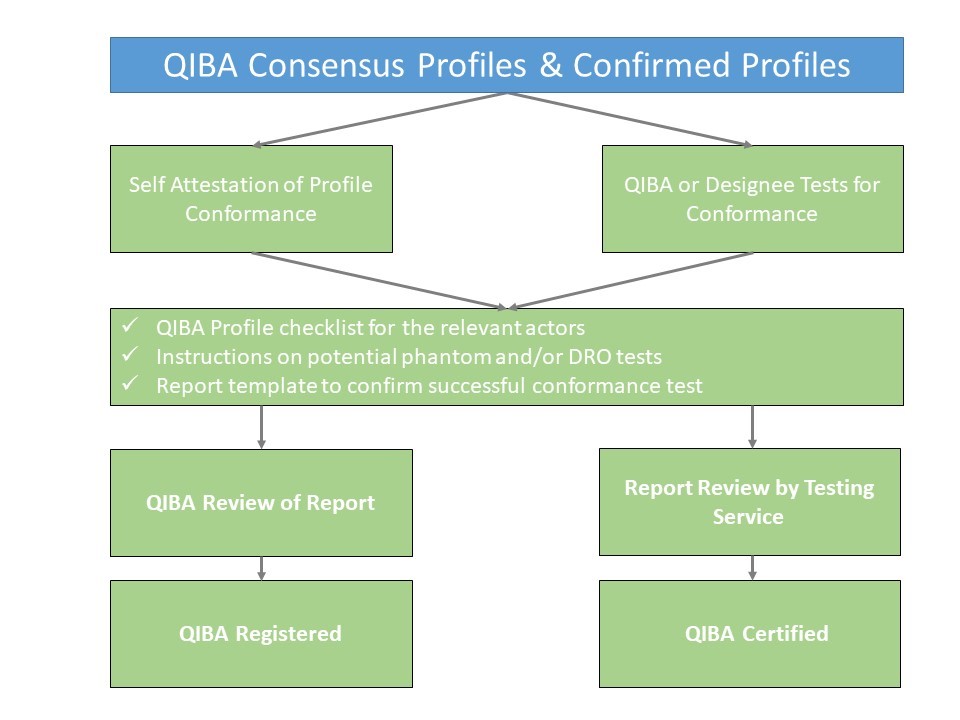
QIBA Profiles that are at least at consensus level (link to wiki page on development of a Profile) are available to interested parties (clinical sites, CROs, equipment and/or software vendors) to demonstrate that all relevant actors of an institution or product conform to the respective QIBA Profile. There are two different ways to show QIBA Profile conformance:
- Self – attestation
- Certification service
The basic process flow is shown below:
Before starting conformance testing, please Email QIBA
Self Attestation of Conformance
The following QIBA Profiles have reached the Consensus Stage or the Technically Confirmed Stage, and are thus appropriate for self-attestation conformance testing.
CT: Lung Tumor Volume (Advanced Disease)
- Profile
- CT Tumor Volume Change for Advanced Disease (CTV-AD) 2018-06-22
- Checklist
- CT Conformance Checklist (can also refer to Appendix C in the Profile document - pg 42)
- Assessment Procedures (refer to Section 4 in the Profile document - pg 30)
- Report Template
FDG-PET: Solid Tumor SUV
- Profile
- FDG-PET/CT for Response to Cancer Therapy 2016-11-18
- Checklist
- QIBA FDG-PET/CT Conformance Testing Checklist, 2019-11-11
- Report Template
MR: ADC-DW MR Lesions in Four Organs
- Profile
- Diffusion-Weighted Magnetic Resonance Imaging Profile 2019-02-05
- Checklist
- QIBA DWI-MRI Conformance Checklist, 2019-11-11
- Report Template