Difference between revisions of "Pilot Conformance Projects Under Development"
Jump to navigation
Jump to search
m |
m |
||
| Line 43: | Line 43: | ||
| − | == <font color=purple>'''QIBA Profiles that are available for QIBA or designee testing:==<font | + | ==<font color=purple>'''QIBA Profiles that are available for QIBA or designee testing:'''==<font> |
*[[Media:QIBA_CT_Vol_SmallLungNoduleAssessmentInCTScreening_2018.11.18-clean-2.pdf|CT Small Lung Nodule Volume Assessment and Monitoring in Low Dose Screening 2018-11-18]] | *[[Media:QIBA_CT_Vol_SmallLungNoduleAssessmentInCTScreening_2018.11.18-clean-2.pdf|CT Small Lung Nodule Volume Assessment and Monitoring in Low Dose Screening 2018-11-18]] | ||
*https://www.rsna.org/en/research/quantitative-imaging-biomarkers-alliance/qiba-conformance-certification-services | *https://www.rsna.org/en/research/quantitative-imaging-biomarkers-alliance/qiba-conformance-certification-services | ||
Revision as of 18:23, 13 November 2019
QIBA Profile Conformance
{This content will eventually be available on the RSNA.org website.}
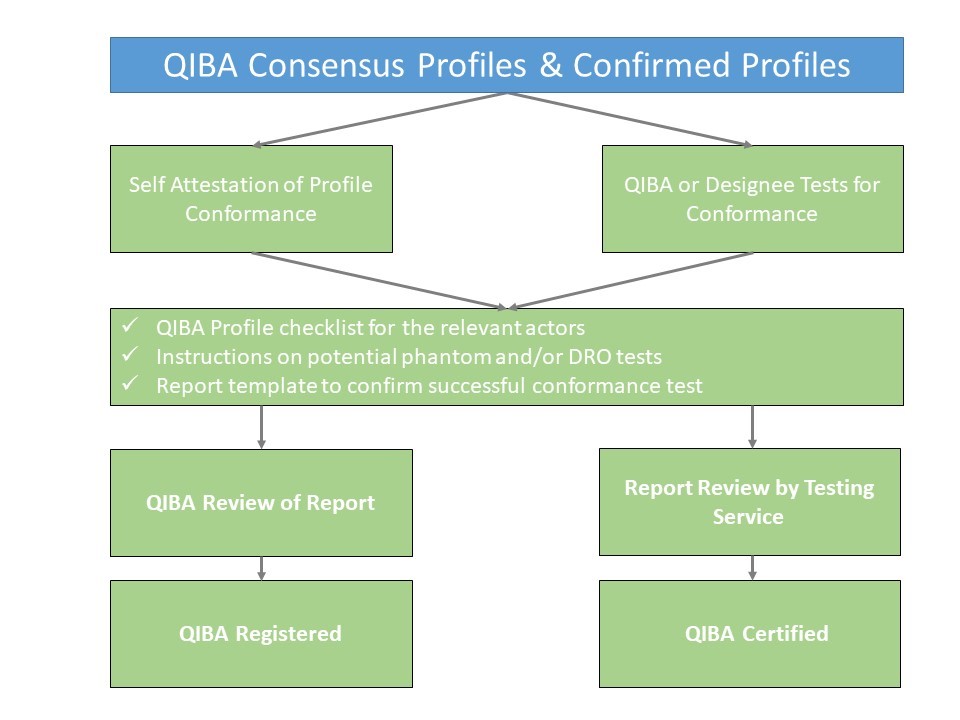
QIBA Profiles that are at least at consensus level (link to wiki page on development of a Profile) are available to interested parties (clinical sites, CROs, equipment and/or software vendors) to demonstrate that all relevant actors of an institution or product conform to the respective QIBA Profile. There are two different ways to show QIBA Profile conformance:
- Self – attestation
- Certification service
The basic process flow is shown below:
Before starting conformance testing, please Email QIBA
QIBA Profiles that are available for self-attestation conformance testing:
CT: Lung Tumor Volume (Advanced Disease)
- Profile
- CT Tumor Volume Change for Advanced Disease (CTV-AD) 2018-06-22
- Checklist
- CT Conformance Checklist (can also refer to Appendix C in the Profile document - pg 42)
- Assessment Procedures (refer to Section 4 in the Profile document - pg 30)
- Report Template
FDG-PET: Solid Tumor SUV
- Profile
- FDG-PET/CT for Response to Cancer Therapy 2016-11-18
- Checklist
- QIBA FDG-PET/CT Conformance Testing Checklist, 2019-11-11
- Report Template
MR: ADC-DW MR Lesions in Four Organs
- Profile
- Diffusion-Weighted Magnetic Resonance Imaging Profile 2019-02-05
- Checklist
- QIBA DWI-MRI Conformance Checklist, 2019-11-11
- Report Template
==QIBA Profiles that are available for QIBA or designee testing:==