Difference between revisions of "Pilot Conformance Projects Under Development"
Jump to navigation
Jump to search
m |
|||
| (32 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ||
| + | |||
| + | == <font color=green>'''QIBA Profile Conformance'''</font> == | ||
| + | <font color=blue>{''This content will eventually be available on the RSNA.org website''.}</font> | ||
| + | |||
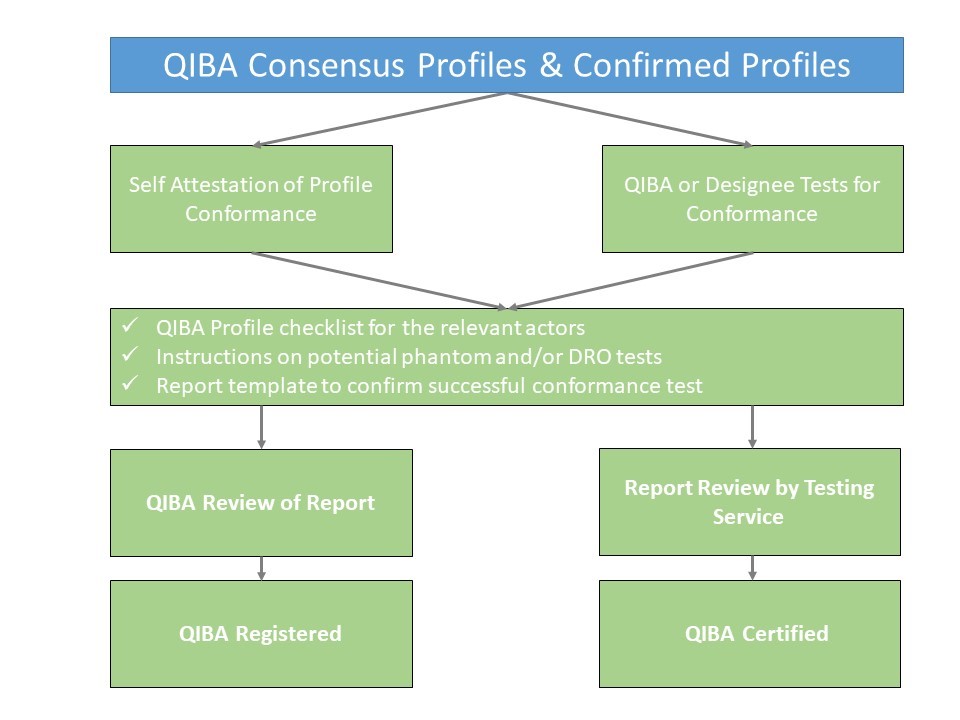
| + | QIBA Profiles that are at least at consensus level (link to wiki page on development of a Profile) are available to interested parties (clinical sites, CROs, equipment and/or software vendors) to demonstrate that all relevant actors of an institution or product conform to the respective QIBA Profile. | ||
| + | There are two different ways to show QIBA Profile conformance: | ||
| + | *Self – attestation | ||
| + | *Certification service | ||
| + | |||
| + | ''The basic process flow is shown below:'' | ||
| + | |||
| + | ::[[Image:Profile Conformance Image.jpg|600px]] | ||
| + | |||
| + | Before starting conformance testing, please [mailto:qiba@rsna.org Email QIBA] | ||
| + | |||
| + | QIBA Profiles that are available for self-attestation conformance testing: | ||
==CT: Lung Tumor Volume (Advanced Disease)== | ==CT: Lung Tumor Volume (Advanced Disease)== | ||
| Line 5: | Line 21: | ||
* [[Media:QIBA_CTVol_TumorVolumeChangeProfile_TechConfirmed-20180622a.pdf|CT Tumor Volume Change for Advanced Disease (CTV-AD) 2018-06-22]] | * [[Media:QIBA_CTVol_TumorVolumeChangeProfile_TechConfirmed-20180622a.pdf|CT Tumor Volume Change for Advanced Disease (CTV-AD) 2018-06-22]] | ||
*'''Checklist''' | *'''Checklist''' | ||
| − | :* [[Media: QIBA_CTVol_TumorVolumeChangeProfile-Conformance Testing_Checklist_20191111 | CT Conformance Checklist]] (can also refer to Appendix C in the Profile document - pg 42) | + | :* [[Media: QIBA_CTVol_TumorVolumeChangeProfile-Conformance Testing_Checklist_20191111.pdf | CT Conformance Checklist]] (can also refer to Appendix C in the Profile document - pg 42) |
:* Assessment Procedures (refer to Section 4 in the Profile document - pg 30) | :* Assessment Procedures (refer to Section 4 in the Profile document - pg 30) | ||
| + | :**[[Media:QIBA_CTVol_TumorVolumeChangeProfile-Conformance Testing_Supplement_2019051111.pdf | CT Conformance Testing Supplement, 2019-11-11]] | ||
*'''Report Template''' | *'''Report Template''' | ||
==FDG-PET: Solid Tumor SUV== | ==FDG-PET: Solid Tumor SUV== | ||
| − | *Profile | + | *'''Profile''' |
| − | *Checklist | + | * [[Media:QIBA_FDG-PET_Profile_v113.pdf|FDG-PET/CT for Response to Cancer Therapy 2016-11-18]] |
| − | *Report Template | + | *'''Checklist''' |
| + | * [[Media:QIBA FDG PETCT _Conformance Testing_Checklist_20191111.pdf | QIBA FDG-PET/CT Conformance Testing Checklist, 2019-11-11]] | ||
| + | *'''Report Template''' | ||
==MR: ADC-DW MR Lesions in Four Organs== | ==MR: ADC-DW MR Lesions in Four Organs== | ||
| − | *Profile | + | *'''Profile''' |
| − | *Checklist | + | *[[Media:QIBADWIProfile_as_of_2019-Feb-05.pdf|Diffusion-Weighted Magnetic Resonance Imaging Profile 2019-02-05]] |
| − | *Report Template | + | *'''Checklist''' |
| + | *[[Media:QIBA DWI Profile _Conformance Testing_Checklist_20191111.pdf | QIBA DWI-MRI Conformance Checklist, 2019-11-11]] | ||
| + | **[[Media:QIBA DWI Profile _Conformance Testing_Supplement 1_20191111.pdf | QIBA DWI Conformance Testing Supplement #1, 2019-11-11]] | ||
| + | **[[Media:QIBA DWI Profile _Conformance Testing_Supplement 2_20191111.pdf | QIBA DWI Conformance Testing Supplement #2, 2019-11-11]] | ||
| + | *'''Report Template''' | ||
| + | |||
| + | |||
| + | ==QIBA Profiles that are available for QIBA or designee testing:== | ||
| + | *[[Media:QIBA_CT_Vol_SmallLungNoduleAssessmentInCTScreening_2018.11.18-clean-2.pdf|CT Small Lung Nodule Volume Assessment and Monitoring in Low Dose Screening 2018-11-18]] | ||
| + | *https://www.rsna.org/en/research/quantitative-imaging-biomarkers-alliance/qiba-conformance-certification-services | ||
Revision as of 22:40, 12 November 2019
QIBA Profile Conformance
{This content will eventually be available on the RSNA.org website.}
QIBA Profiles that are at least at consensus level (link to wiki page on development of a Profile) are available to interested parties (clinical sites, CROs, equipment and/or software vendors) to demonstrate that all relevant actors of an institution or product conform to the respective QIBA Profile. There are two different ways to show QIBA Profile conformance:
- Self – attestation
- Certification service
The basic process flow is shown below:
Before starting conformance testing, please Email QIBA
QIBA Profiles that are available for self-attestation conformance testing:
CT: Lung Tumor Volume (Advanced Disease)
- Profile
- CT Tumor Volume Change for Advanced Disease (CTV-AD) 2018-06-22
- Checklist
- CT Conformance Checklist (can also refer to Appendix C in the Profile document - pg 42)
- Assessment Procedures (refer to Section 4 in the Profile document - pg 30)
- Report Template
FDG-PET: Solid Tumor SUV
- Profile
- FDG-PET/CT for Response to Cancer Therapy 2016-11-18
- Checklist
- QIBA FDG-PET/CT Conformance Testing Checklist, 2019-11-11
- Report Template
MR: ADC-DW MR Lesions in Four Organs
- Profile
- Diffusion-Weighted Magnetic Resonance Imaging Profile 2019-02-05
- Checklist
- QIBA DWI-MRI Conformance Checklist, 2019-11-11
- Report Template